Understanding the Plasticity of Diamond for Improved Fusion Ignition
September 20, 2023
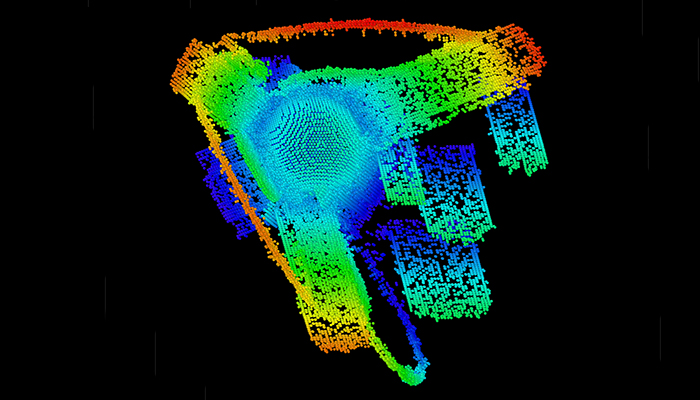 When a diamond undergoes plastic deformation, atomic bonds break and re-form along imperfections called dislocations. Taken from a molecular dynamics simulation, this rendering represents a shocked diamond as a pore collapses under pressure. Each dot represents a defective atom within the diamond’s crystalline structure, while the different colors indicate the longitudinal position of the dislocations.
When a diamond undergoes plastic deformation, atomic bonds break and re-form along imperfections called dislocations. Taken from a molecular dynamics simulation, this rendering represents a shocked diamond as a pore collapses under pressure. Each dot represents a defective atom within the diamond’s crystalline structure, while the different colors indicate the longitudinal position of the dislocations. Alex Li, an LLNL summer student in the Computational Chemistry and Materials Science Summer Institute, recently led a study to investigate the evolution of plasticity in diamond. The nature of this deformation is important for high energy density experiments on high-energy laser systems such as NIF, as well as for furthering scientific understanding of carbon-rich exoplanets.
While diamond carbon is one of nature’s strongest naturally occurring materials, it is known to undergo irreversible plastic deformation when loaded, or stressed, at high rates.
“We usually think of diamond as brittle, strong, and unyielding until it cleaves,” said LLNL scientist Rob Rudd, Li’s mentor. “Shocked diamond is different. Alex did a fantastic job working out the complex and unexpected details of the deformation.”
The team’s research investigated the plasticity in diamond along different loading orientations and the effects that voids (pores) within the material can have on stresses within the diamond.
The research, published in the journal Matter, was supported by LLNL’s Laboratory Directed Research and Development program.
At NIF, diamond carbon is used as an ablator and capsule material for producing the extremely high pressures needed to cause nuclear fusion reactions that are being intensively investigated for stockpile stewardship and as a source of energy.
In many shock-compression experiments, diamond has shown little to no plastic behavior until reaching extreme pressure and temperature conditions. Because diamond exhibits strong anisotropic behavior—that is, it has different properties in different locations, depending on the direction of applied stress—it can be difficult to anticipate how diamond will react under such extremes. Defects present within the diamond capsules that hold NIF’s fusion fuel can cause imperfect compression, resulting in a failure to ignite.
Using molecular dynamics and analytical calculations on three diamond orientations under shock compression, Li’s team demonstrated that both the stress needed to generate defects and the character of the defects depend on the orientation.
In addition to computer simulations, the team used the Omega Laser Facility at the University of Rochester’s Laboratory for Laser Energetics to shock diamonds under extreme loading conditions and recover them for subsequent microscopic analysis. Omega’s high laser energy enabled the imposition of the extreme stress- and strain-rate conditions. Stress refers to the force applied to a material per unit area, while strain refers to the deformation or change in the material’s shape resulting from the applied force.
Of the three orientations tested, two showed significantly more defect activity, with even more defects being generated when a void was intentionally introduced. Building a better understanding of diamond’s behavior under shock loading can help researchers optimize the diamond capsules used at NIF, suppressing undesired effects such as instabilities and leading to the development of better models or capsules to get closer to realizing fusion as an energy source.
—Shelby Conn
Follow us on Twitter: @lasers_llnl



